Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
斉藤 淳一; Monbernier, M.*
Surfaces and Interfaces (Internet), 41, p.103248_1 - 103248_8, 2023/10
被引用回数:0 パーセンタイル:0.01(Chemistry, Physical)Contact angle is an indicator of the wettability between liquid Na and pure metals. This has been evaluated using the atomic interactions obtained from the calculations of the electronic structure of the interface. This study aims to investigate the applicability of the atomic interactions of the interface to the alloys. An interface model between Cu and Na with an alloying element was constructed, and the electronic states of the interface were calculated by the molecular orbital calculation. The bond order, which indicates the strength of the covalent bonding at the interface, and ionicity, which indicates the amount of charge transfer, were obtained as theoretical parameters from the calculation. The contact angles between the Cu or Cu alloys and liquid Na were measured using a droplet of liquid Na at 423 K in a high-purity Ar atmosphere. The contact angles of the Cu alloys were evaluated using these theoretical parameters. As a result, a correlation was obtained between the ratio of the bond order between the substrate metal atoms to the bond order between the Na atom and the substrate metal atoms and the contact angle, which is consistent with previous studies. Furthermore, for the first time, the correlation between the ionicity or difference in the ionicity and contact angle was clarified. The difference in ionicity is the difference between the ionicity of Na atoms and that of the alloying element, indicating the strength of the ionic bonding. It was suggested that Cu and Cu alloys should consider covalent and ionic bonding when evaluating wettability, because Cu has an intermediate electronic state between transition and nontransition metals. Further, it became clear that the evaluation of the contact angle using the atomic interactions at the interface are applicable not only to pure metals but also to alloys.
鈴木 康之*; Li, J.; 前川 康成; 吉田 勝; 前山 勝也*; 米澤 宣行*
日本化学会誌, 2002(2), p.255 - 259, 2002/02
PET膜へのイオンビーム照射により作製したイオン穿孔膜は、直径が0.01-10 mと、膜厚に対する孔径が小さく、孔径分布が狭い微細孔を有するため、機能性膜などの応用が期待できる。このイオン穿孔膜は加水分解により得られるため、機能性膜へ適用する場合、その親水性表面の安定性が重要となる。そこで、加水分解後のPETを乾燥空気下,窒素下,飽和蒸気下、及び減圧下,0
mと、膜厚に対する孔径が小さく、孔径分布が狭い微細孔を有するため、機能性膜などの応用が期待できる。このイオン穿孔膜は加水分解により得られるため、機能性膜へ適用する場合、その親水性表面の安定性が重要となる。そこで、加水分解後のPETを乾燥空気下,窒素下,飽和蒸気下、及び減圧下,0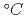 から80
から80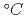 で放置し、膜表面の接触角変化から、その安定性に及ぼす環境と温度の影響を評価した。加水分解により得られたPET表面の疎水性への変化は、親水的環境である飽和水蒸気下で全体的に著しく速くなった。親水性PET表面の各環境下でのcos
で放置し、膜表面の接触角変化から、その安定性に及ぼす環境と温度の影響を評価した。加水分解により得られたPET表面の疎水性への変化は、親水的環境である飽和水蒸気下で全体的に著しく速くなった。親水性PET表面の各環境下でのcos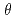 の変化速度を疎水加速度と定義し、速度定数kの温度依存性について調べた結果、飽和水蒸気下では、他の条件と比較して温度依存性が高いことがわかった。したがって、親水的環境での疎水化の促進は、PET表面への水分子の吸着により高分子膜表面の運動性が上昇したため、親水性基の内部への拡散が速くなったためと結論した。
の変化速度を疎水加速度と定義し、速度定数kの温度依存性について調べた結果、飽和水蒸気下では、他の条件と比較して温度依存性が高いことがわかった。したがって、親水的環境での疎水化の促進は、PET表面への水分子の吸着により高分子膜表面の運動性が上昇したため、親水性基の内部への拡散が速くなったためと結論した。
川久保 昌平*; 大野 宏和; 松井 裕哉; 加藤 猛士*
no journal, ,
国内外において、ボアホールジャッキ試験による岩盤の変形係数は、実際の値よりも過小評価される傾向にあることが指摘されている。その要因として、高圧時の載荷板の曲げや孔壁と載荷板の曲率不一致が挙げられている。これらの影響は既に海外で研究され、その補正方法が提案されているが、対象とするボアホール径や試験器は海外規格のものであり、載荷圧も国内で行われる試験に比べて高いレベルを想定している。このため、我が国で岩盤を対象としてボアホールジャッキ試験を行うにあたり、変形係数の適切な評価のための補正方法は未だ存在しない。そこで、本研究では国内で使用される試験器の規格・仕様等を踏まえ、孔壁と載荷板の密着性が低下した場合の変形係数算定における補正方法を提示するものである。
小林 洋平*; 斉藤 淳一; 澁谷 秀雄*
no journal, ,
Wettability is one of properties between liquid and solid material and it is very important factor affecting on acoustic property, corrosion property and so on. In this study, some experiments of wettability of pure metals were performed using the low-melting temperature alloy in order to get many fundamental information of wettability. The low-melting temperature alloy consists of bismuth, tin and indium. Aluminum, titanium, iron, nickel, copper, zirconium, niobium and molybdenum were used as a substrate metal. Test temperature was 353K and test atmosphere was in air. A droplet of the low-melting temperature alloy was put on the substrate metal and a contact angle was measured. From the measurement results, the contact angle changed depending on the substrate metal. It is suggested that the contact angle has relations with the atomic bonding between the s
斉藤 淳一; 小林 洋平*
no journal, ,
The purpose of this study deeply understands of wettability from the relationship between the contact angle and the atomic bond of the interface by using the result of theoretical calculation. As the substrate metal, nine kinds of pure metals and alloys were used. All of the contact angle were smaller than 90 degrees. It means the wettability of water with metal is good. The contact angle results have been evaluated using the bond order, which shows the strength of atomic interaction at the interface. For liquid metals and metals, the contact angle could be explained by the atomic bond at the interface. It was found that there is a good correlation between the contact angle and the atomic bond without contradictory to Young's equation even if the atomic bond at the interface is used. This suggests that it is possible to predict the wettability of an unknown metal with water.